
The polymerase chain reaction (PCR, described here) works mainly because of two components – a heat-stable DNA polymerase enzyme (adds new nucleotides to a chain of nucleotides) and a pair of DNA PCR primers.
The PCR aims to detect the presence of a section of genetic material; usually only a small part of a greater whole. Its detection is used to infer the presence of a mutation, genetic fragment, gene, disease, person or pathogen. It relies on genetic sequence recognition and on the fact that genetic sequences are unique to an entity whether its a human, chimp, cabbage or virus.
Sometimes PCR designs make use of the similarities between members of the same genetic groups, sometimes the design will seek to pick out a particular genetic subset of a wider related grouping. For example a specific coronavirus (e.g. SARS-CoV-2) from among the wealth of coronaviruses out there.
PCR permits very
PCR is a great example of biology, physics and chemistry colliding. I’ll try and keep it simple but feel free to ask questions in eh comments if this is confusing but you want to understand it.
What is a primer…?
Primers are short, made-to-order, stretches of DNA called oligonucleotides (‘oligos‘ – from Greek meaning scanty or few). Modern oligos (the shorthand term) can be synthesized in lengths >100nt however the behaviour of oligonucleotides longer than 20nt is different from that of shorter oligos and different calculations are employed to determine their thermodynamic characteristics.
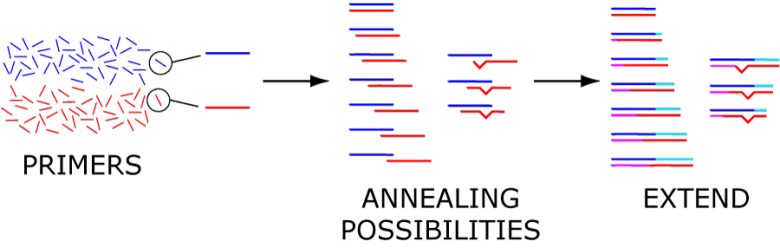
A primer, as its name may suggest, primes a target nucleic acid sequence for the attachment of a polymerase – the DNA strand-making enzyme. This is the first step towards duplicating that target region. The primer directs the polymerase to move in a 5′ to 3′ direction (drawn left-to-right; Figure 1) because of the ‘direction’ of DNA (See DNA Structure for more background).

For the best PCR amplification, PCR primers occur in pairs – a forward and a reverse primer, also called sense and antisense, respectively. These sit apart from each other, facing toward each other and spanning the genetic region to be copied. Each copy of the target will incorporate each primer at its ends, or termini.
The exponential production of those millions of copies of a region requires a back and forth copying of that target region. Ideally, the primer pair has been designed so that both primers work well during the thermal cycling process.
Primers can be designed for different uses
When designing a new PCR (or RT-PCR, depending on whether your target is DNA or RNA), you may want to detect a very specific virus from among many related viruses (SARS-CoV-2 but not SARS-CoV for example). Or you may want to be able to detect all the viruses in a species or genus or family. These designs may sometimes be called “universal” primers – but that’s a case-by-case thing. It can be nearly impossible to design good universal primers because the genetic variation between viruses is often too great.
Sometimes you may find that a bunch of viruses in the same species/genus/family/etc have a genetic difference at one of the nucleotide positions you want your primer to land on. These “wobbles” can be accounted for by designing multiple primers – each binding to the same landing site – but each with the relevant nucleotide in that position. Or you can design a “degenerate” primer – one primer (actually still a mix) that uses a code to basically do the same thing, but tells the manufacturer what sort of wobble you want.
There are also special chemically modified nucleotides that can be include in primers to enhance binding or decrease specificity.
Primer binding…
Primers hybridize, or “bind”, at a temperature that is affected by their sequence, concentration, length and ionic environment. This annealing temperature is usually referred to as the TM (melting temperature) but is usually 5–10°C below the TM. The term TM describes the temperature at which 50% of the primer–target duplexes have formed, and 50% have not.
When designing a primer pair, we make sure that TM of both primers is similar so they work well at the chosen TM.
PCR can be very specific to its target because of the primers…
PCR gleans its extreme specificity from the primers. At each and every position of a new primer, we have 4 nucleotides to choose from, dATP, dCTP, dGTP and dTTP.

So, if we design a sequence-specific primer of 20-30nt nucleotides in length (’20-30mer’), the chance that that exact sequence will occur randomly in nature will be 1/4 x 1/4 x 1/4 etc, 20 or 30 times i.e.

That means a 1 in 1012 to 1018 chance of a 100% homologous match to an unintended target. Or to put that in perspective, there are 2.85 x 109 base-paired nucleotides in the entire human genome.
While that all sounds very convincing, in reality, primers designed to detect viruses often share significant amounts of homology with the human genome – sometimes resulting in false-positive amplification. Even when the homology is far from 100%, primers may still amplify an unintended target as shown below. This most likely reflects the co-evolution of many viruses with humans during which time they have “captured” bits of our genome and “deposited” bits of their own genome.
Next, we’ll list a few of the problems we can encounter when using the PCR.
Primer dimer…
The first problem I’ll discuss is the most common and the most difficult to avoid. Depending on your requirements, it may also be the least significant.
When a small amplicon results from the extension of self-annealed primers, you get primer-dimer (PD) i.e. a dimer of one (self-annealing) or both primers resulting in a template capable of being extended by the polymerase. PD formation is highly efficient because the primers are in vast excess compared to the amount of template or even to the number of amplicon molecules at the end of the PCR.
This excess drives the formation of PD. Two main concerns arise from PD formation.
- Because PD formation is so efficient, it rapidly consumes dNTPs and primers and generates amplification inhibiting pyrophosphates. All of which can prematurely plateau the exponential accumulation of product.
- If we using a dsDNA-associating fluorescent molecule to follow the PCR’s progress during real-time PCR, then PD will also show up, and, at least during the kinetic portion of the assay, cannot be differentiated from the signal of specific amplicon accumulation.
Ten examples are shown of sense and antisense primer interactions resulting in an amplicon. Note that different length amplicons can be formed.
The largest PD would result from the hybridization of the smallest number of nucleotides and would approach the length of the two primers added together.
Mispriming…
Mispriming of PCR primers is the result of a primer binding to an unintended template resulting in amplification. The amplicon (PCR product of a single species) can sometimes be the same size as the intended product but is usually a different size when viewed following agarose gel electrophoresis.
Mispriming occurs because of poorly optimised conditions or because we haven’t checked whether our sequence will inadvertently bind to an entirely different target entity e.g. a region of the human genome instead of the intended virus genome. Sometimes it just happens.
Mispriming can usually be avoided by more intensive comparison of the primer’s sequence against the GenBank database using the Basic Local Alignment Search Tool (BLAST) at NCBI. Of course, a BLAST comparison will only find matches among those sequences housed in the database. When it comes to PCR where a single nucleotide mismatch can cause amplification to fail, or at least perform with reduced efficiency, BLAST’ing primers can lead to a feeling of very false security.
In some instances, the homology of the PCR primers to their template may indicate a perfect match simply because viral variants have not yet been sequenced and submitted. Also, because there may be many undiscovered viruses and unsequenced non-viral genomes in the world, obviously none of which are represented on GenBank, a specific match, or a “no match”, does not mean that you have exhausted your search for homologues. Take it all with a grain of salt. Designing two pairs of primers around the target region is a good place to start. This helps address the unexpected.
Structural problems…
This problem results from the way we design our PCR primers. I am excluding self-annealing and secondary structures from here because we will deal with them specifically in another section. —work in progress—
Further reading…
- A crowd-sourced database of virus primers. www.virusprimers.org/
- The mechanics of the polymerase chain reaction (PCR)…a primer
http://virologydownunder.blogspot.com.au/2015/05/the-mechanics-of-polymerase-chain.html - Reverse transcription-polymerase chain reaction (RT-PCR)…a primer
http://virologydownunder.blogspot.com.au/2015/05/reverse-transcription-polymerase-chain.html - Mackay IM. Real-time PCR in the microbiology laboratory. 2004. Clin Microbiol Infect. 10(3):190-212.
- Mackay IM, Arden KE and Nitsche A. 2002. Real-time PCR in virology. Nucleic Acids Res. 30;6. 1292-1305.
- Beld MGHM, Birch C, Cane PA, Carman W, Claas ECJ, Clewley JP, Domingo J, Druce J, Escarmis C, Fouchier RAM, Foulongne V, Ison MG, Jennings LC, Kaltenboeck B, Kay ID, Kubista M, Landt O, Mackay IM, Mackay J, Niesters HGM, Nissen MD, Palladino S, Papadopoulos NG, Petrich A, Pfaffl MW, Rawlinson W, Reischl U, Saunders NA, Savolainen-Kopra C, Schildgen O, Scott GM, Segondy M, Seibl R, Sloots TP, Wang Y-W, Tellier R and Woo PCYl. Chapter 10:”Experts’ roundtable: Real-time PCR and microbiology”, In: Real-Time PCR in Microbiology, IM Mackay (Editor). 2007. Caister Academic Press, Norfolk, UK.
- Mackay IM, Arden KE, Nissen MD and Sloots TP. Chapter 8. “Challenges facing real-time PCR characterisation of acute respiratory tract infections”, In: Real-Time PCR in Microbiology, Mackay IM (Editor). 2007. Caister Academic Press, Norfolk, UK. 269-317.
- Mackay IM, Mackay JF, Nissen MD and Sloots TP. Chapter 1: ”Real-time PCR; History and fluorogenic chemistries”, In: Real-Time PCR in Microbiology, IM Mackay (Editor) 2007. Caister Academic Press, Norfolk, UK.
- Mackay IM, Bustin S, Andrade JM, Kubista M and Sloots TP. Chapter 5: ”Quantification of microorganisms: not human, not simple, not quick”, In: Real-Time PCR in Microbiology, IM Mackay (Editor). 2007. Caister Academic Press, Norfolk, UK.
- Mackay IM, Arden KE and Nitsche A. Real-time fluorescent PCR techniques to study microbial-host interactions. Methods in Microbiology, Microbial Imaging. (2005) Vol 34. Chapter 10.Elsevier. pp255-330.
- Mackay IM. Respiratory viruses and the PCR revolution. In: PCR Revolution: Basic technologies and applications, Bustin, SA (Editor). 2010. Ch 12. Pp189-211. Cambridge University Press.
*Imported Post
- This post from 05MAY2015 was posted over on my old blog platform virologydownunder.blogspot.com.au. It has now been moved to here.
Edits
- 06MAY2020. Added more detail about PCR and different primer types and what a probe is.
Views: 1358
